افشین رشید
اُستادیار ؛ عضو هیات علمی دانشگاه آزاد اسلامی واحد علوم و تحقیقات تهران
651 یادداشت منتشر شدهDetailed investigation (Nanoparticle Size and Electronic Conduction Properties) in Nanofullerenes
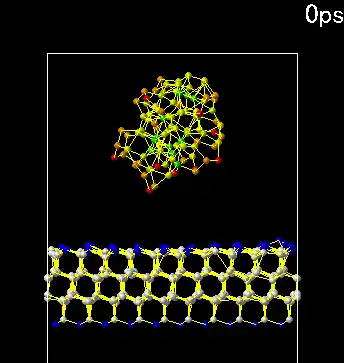
Note: An important characteristic of all nanostructures is that the number of surface atoms in them is greater than the number of bulk atoms. This ratio increases as the size of the nanoparticle decreases. Therefore, the size of the nanoparticle is considered its important characteristic.
The range of activity variation of nanoparticles depends on the nature and shape of the nanostructure. However , if the energy of the nanoparticle field is comparable to the energy of the electromagnetic radiation and if significant changes occur in the irradiated material within a certain wavelength range due to chemical reactions, the activity of nanoparticles up to 100 nm will be significant. The atoms on the surface of the nanoparticles are not compensated for in terms of energy. In general, the results of the energy growth of the nanoparticle can be expressed as the total energy of the atoms on the surface of the particle.
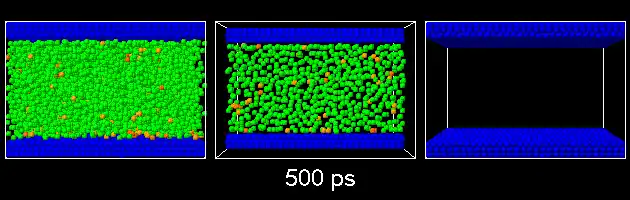
The freedom of movement of atoms on the surface of nanostructures is limited and only vibrational movements and electron motion are possible. These two electrokinetic reactions are interdependent because the displacement of the electron clouds of atoms necessarily changes the vibrational frequencies of the bonds of the atoms of the nanoparticles . On the other hand, the displacement of the valence electrons in the bonds changes the polarity of the bond and the bodies called the supermolecule. In this case, the electron transfer to a higher energy level becomes possible. In this respect, carbon nanotubes (CNTs) are the most interesting species under investigation. In these carbon nanotubes, which protect them from the environment, interact and for this reason these CNTs are called metal/carbon nanotubes.
Fullerenes are identified by the number of atoms in their structure. Fullerenes are named by the letter C, which represents the carbon atom in their structure. The letter C is followed by the number of carbon atoms in the fullerene's spherical lattice unit. For example, the C60 molecule has 60 carbon atoms. The number of atoms in fullerenes produced so far ranges from 28 to hundreds of carbon atoms.
Conclusion :
An important characteristic of all nanostructures is that the number of surface atoms is greater than the number of bulk atoms. This ratio increases as the size of the nanoparticle decreases. Therefore, the size of the nanoparticle is considered an important characteristic.