افشین رشید
اُستادیار ؛ عضو هیات علمی دانشگاه آزاد اسلامی واحد علوم و تحقیقات تهران
651 یادداشت منتشر شدهElectronic structure and stability of Fullerene C۸۰ isomers
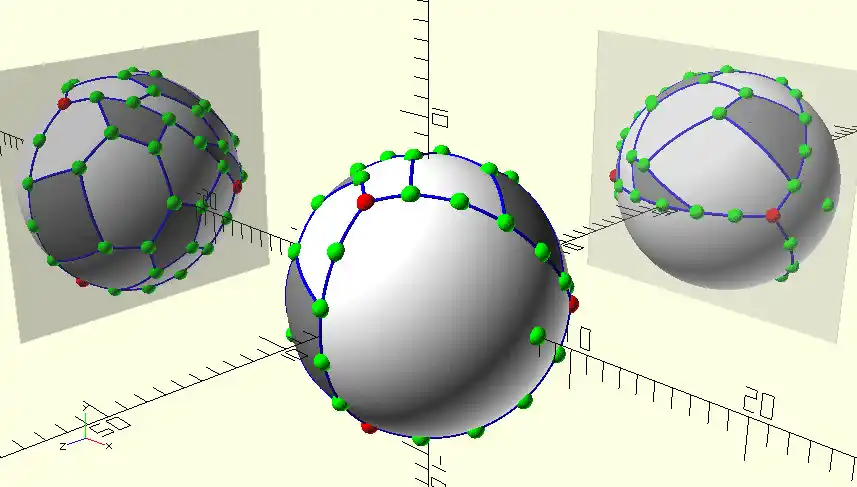
Note: The distribution of π, single, double, and delphilation bonds in the molecules of the seven isomers of fullerene C 80 in radical substructures is the reason for the molecular instability of unstable isomers and the increase of electromagnetic properties in nanotubes.
The hexagonal and pentagonal distortions are shown to be the properties of the molecules of all Fulleren C80 isomers , but the maximum distortion, similar to C 70 , occurs in the hexagon with the separated P -bonds, which affect the stability and electronic properties of Fulleren. Does not affect. Bucky Ball (c80) and fullerenes are some of the materials on which many nanomaterials are based. Gone in specific electrical circuits, nanophotonics in solar nanocells and nano-absorbers are specific wavelengths. One of the most important and unique properties of fullerenes is their ability to retain atoms or small molecules inside the carbon cage. Endo-Federal Carbon Cages (EFs), due to their inherent stability,Their endodontic nanostructures are synthesized, containing one or two metal atoms such as Sc and La, or containing a metal-cluster nitride such as Sc3N . The importance of spatial and electronic parameters related to cage structure such as fullerenes in the factors of this importance is the introversion of nanostructures and the stability of these compounds. The optical properties, ionization and electron-seeking energies, and the relative stability of the various polygons of fluorine are very influential in the proliferation of nano-electronic devices.

Fullerens are identified by the number of atoms in their structure. A letter C is used to name fullerenes, indicating the carbon atom in their structure. After the letter C, the number of carbon atoms in the Fullen spherical lattice unit is stated. For example, the c80 molecule has 80 carbon atoms. The number of atoms produced in fullerenes so far ranges from 28 to hundreds of carbon atoms.
Conclusion:
An important feature of all nanostructures Fullerene C80 A Nest in the number of surface atoms relative to the number of atoms in it more volume. This ratio increases in C80 fluorescents by decreasing the nanoparticle size. Therefore, the nanoparticle size of c80 is an important feature.