افشین رشید
اُستادیار ؛ عضو هیات علمی دانشگاه آزاد اسلامی واحد علوم و تحقیقات تهران
651 یادداشت منتشر شده_ Simulation process section in (Electrical Nanoparticles) Nano Molecular drift to stabilize nanoparticles in the simulation process
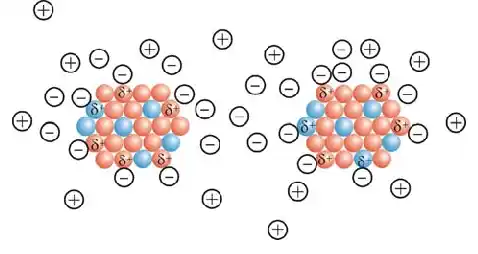
Note: Due to their high specific surface area and surface energy, the propagated nanoparticles stick together and form aggregates. This phenomenon leads to the loss of the properties resulting from the small size of these particles.
To prevent the accumulation of nanoparticles during the synthesis stage, stabilizers are used. Usually, two types of methods are used to stabilize nanoparticles: electrostatic and steric drift. In the first method, ions are used to stabilize nanoparticles. These ions are attracted to the particles and form an electrically charged layer around the nanoparticles, and as a result of molecular drift, the nanoparticles produced stick together and form a mass due to their specific surface area and high surface energy. This phenomenon leads to the loss of properties resulting from the small size of these particles. To prevent the accumulation of nanoparticles during the synthesis stage, stabilizers are used.
Typically, two methods are used to stabilize nanoparticles: electrostatic and steric drift. Here is a sample of two methods for stabilizing particles. In the first method, ions are used to stabilize nanoparticles. These ions are attracted to the particles and form an electrically charged layer around the nanoparticles, resulting in molecular drift from particle aggregation.

In the second method, macromolecules are used to stabilize nanoparticles. Macromolecules adhere to the surface of the particles and occupy space around the particle. As the particles approach each other, these molecules become entangled and prevent the particles from sticking together, resulting in particle agglomeration. In the second method, macromolecules are used to stabilize nanoparticles. Macromolecules adhere to the surface of the particles and occupy space around the particle. As the particles approach each other, these molecules become entangled and prevent the particles from sticking together. In the propagation of nanoparticles by electrostatic reduction, the molecular drift method is usually used to stabilize the particles. One of the parameters that greatly affects the size in the electrostatic synthesis of nanoparticles is the concentration of the precursor. The higher the concentration of the precursor, the larger the size of the particles produced, and conversely, the lower the concentration of the precursor , the smaller the size of the particles.
Conclusion :
The resulting nanoparticles are stabilized by various stabilizers. Depending on the electrostatic and molecular drift conditions in the nanoparticles, each stabilizer can have advantages over other stabilizers in the propagation of nanoparticles. To prevent the accumulation of nanoparticles, the concentration of stabilizers must be within a certain range. Too low a stabilizer concentration cannot prevent the accumulation of nanoparticles, and on the other hand, too high a stabilizer concentration interferes with the production of nanoparticles.
- Nanoelectronics and the effect of quantum confinement Nanoparticle fabrication and the effect of quantum confinement
🔬 Biological _ Electrical Nanosensors Section Investigation of biological-electrical nanosensors (Nano biosensors), such as the senses of smell and taste, which are used to identify different smells and tastes.