افشین رشید
اُستادیار ؛ عضو هیات علمی دانشگاه آزاد اسلامی واحد علوم و تحقیقات تهران
615 یادداشت منتشر شدهBuckminsterfullerene explanation from the point of view of nanoscience and nanoelectronics
Note: Carbon nanotubes have many uses in nanoscience and nanoelectronics. Carbon is one of the amazing elements of nature that can be found in nature in four different forms: graphite, diamond, nanotubes and buckyballs. All these four forms are solid and in their structure, carbon atoms are completely and regularly placed next to each other.
Carbon is one of the most important elements in nature, and its many uses in human life confirm this point well. For example, steel - which is one of the main engineering alloys - is obtained from the dissolution of about two percent of carbon in iron; Different types of steel can be obtained by changing the carbon percentage by only a few hundred percent. "Organic chemistry" is also a science that examines compounds containing "carbon" and "hydrogen" and polymer engineering is based only on the carbon element. Carbon is found in four different forms in nature, all of these four forms are solid, and in their structure, carbon atoms are placed next to each other in a completely regular manner.
These structures are:
01. Graphite
02. Diamond
03. (bucky wings) like C60
04. Nanotubes
Graphite is one of the most important carbon structures in nature, and it is formed by placing six carbon atoms next to each other. These carbon atoms are combined with each other in such a way that they create a regular hexagon and from their sum, a sheet is obtained which is considered as a (graphite layer).
Carbon atoms are connected to each other by covalent bonds - which is a strong and strong bond. It should be noted that carbon atoms used in a graphite layer cannot bond covalently with carbon outside this layer. Therefore, a graphite layer is connected to the underlying layer through van der Waals bonds , which are weak bonds. This problem makes the graphite plates easily slide on each other. For this reason, this compound is used in "oiling" and "lubricating". This is also the reason for the smoothness of the surfaces that are written on with a pencil .
Diamond- 1-2
At very high temperatures and pressures, carbon is stable in diamond form, where each carbon atom is covalently bonded to four atoms. Due to the strong covalent carbon-carbon bond, it is considered one of the hardest substances in nature with a hardness of 01 on the Morse scale. This structure has created amazing properties in diamond, among which one can mention the thermal conductivity several times that of copper and the melting temperature of about 3111.
3-1 - Bucky wings
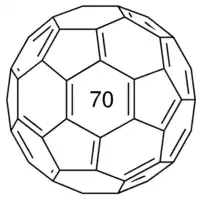
Buckyballs are spherical and hollow carbon structures, the most famous of which is C60 or fullerene. C60 has a structure similar to a soccer ball . Carbon atoms in it are connected by covalent bonds in the form of pentagons and hexagons. In fact, this sphere consists of 30 parts, 01 of which is hexagonal and 00 of which is pentagonal. Bucky wings have many uses due to their chemical properties and their hollow and cage-like shape. Bucky wings are extremely stable and can withstand very high pressures and temperatures. Carbon atoms in the wings can react with atoms and molecules without changing or causing defects in their stability and spherical structure.
4-1 - Carbon nanotubes
Carbon nanotubes are one of the most important and widely used carbon structures that have been discovered recently. They have unique properties and characteristics . In addition to being very strong, carbon nanotubes also have good flexibility and twistability. One of their applications is composite. The most important property of nanotubes is their electrical conductivity, which varies depending on the arrangement of atoms .

Conclusion :
Carbon nanotubes are cylindrical molecules with open or closed ends. The structure of nanotubes is like a rolled sheet of graphite. To better understand the nanotube structure, consider a graphite layer. We locate the atoms that are placed in a row with (m,n) which indicates the coordinates of a point in the plane. so that n coordinate is related to the column of atoms and m coordinate is related to the row of atoms. As we know, to make a tube from a plate, it is enough to place one point of the plate on another point. A nanotube is like a graphite sheet shaped like a tube. Depending on how the two ends of the graphite sheet are connected to each other, we will have different types of nanotubes.