افشین رشید
اُستادیار ؛ عضو هیات علمی دانشگاه آزاد اسلامی واحد علوم و تحقیقات تهران
651 یادداشت منتشر شدهInvestigating The Electronic Storage Capability (Superconductivity) in Carbon Nanotubes and Graphene Particles
Note: In nanotubes, every three carbon atoms are capable of storing one lithium ion, while in graphite, every six carbon atoms are capable of storing one lithium ion. Also, the energy storage capacity of nanotubes is many times the volume of graphite electrodes.
Large amounts of hydrogen can be stored in nanotubes for energy and fuel cell applications. They are superconducting. The radius of these superconducting nanotubes is only 6.4 nm. Phase transitions in one- or two-dimensional systems where room-temperature superconductivity can be found in carbon nanotubes and graphene particles, and superconductivity is present in them. When a liquid passes through coils of single-walled carbon nanotubes, an electrical voltage is generated. This technique is used to make liquid flow sensors to detect very small amounts of liquid and to generate voltage in biomedical applications. Liquids with high ionic strength also produce higher voltages .
The increase in thermal power and resistance of nanotubes is proportional to the cube root of the mass of atoms and molecules. Heating also increases the strength of the nanotube, increasing its tensile strength sixfold and increasing its conductivity. As atoms or molecules collide with a carbon nanotube, its electrical resistance changes.
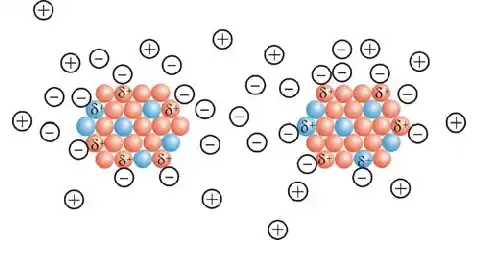
Arc discharge method
In this method, carbon atoms are heated and vaporized by passing a high current through the two electrodes, the anode and cathode, into a helium gas plasma . The electric arc method is the most common and perhaps the simplest method for producing carbon nanotubes, and in it a mixture of nanotubes and impurities is produced, which makes it possible to separate the nanotubes from the soot and metals . The thallium present in the raw product is essential. In this method, nanotubes are produced by electric arc evaporation of two carbon rods that are placed opposite each other and at a distance of approximately 1 mm from each other. The electrodes are placed in a chamber filled with an inert gas (helium or argon) at a relatively low pressure of 56-766 mbar. Creating a potential of about 26 volts with a current in the range of 56-166 amps causes an electrical discharge in the gas at high temperature, and the heat from the discharge evaporates one of the carbon electrodes and a deposit in the form of small rods is formed on the other electrode.
Conclusion :
The ability to store electricity (superconductivity) in carbon nanotubes and graphene particles has made nanotubes an ideal choice for many applications. The existence of a series of special properties of carbon nanotubes has focused their use in the manufacture of devices, and they will have the possibility of making very wide industrial devices.